What Is the Conjugate Base of Oh
H₄N₂ conjugate base Explanation. You get the conjugate base after deprotonation taking a hydrogen atom so it is OHtext-.

180bronsted Gif Chemistry Lessons Apologia Chemistry Teaching Science
What is the conjugate base of HC6H6O6-.

. We see that HCO₃ becomes H₂CO₃. The conjugate base of OH is O 2- as the conjugate base is formed when H is removed from the given species. The weaker the acid or base the stronger theconjugate.
HCO3- known as bicarbonate is the conjugate base of H2CO3 a weak acid and the conjugate acid of the carbonate ionHCO3- acts as a base when mixed with a compound. Conjugate baseOX 2 HX. It has one more H atom and one more charge -1 1 0.
HCO₃ H₂O H₂CO₃ OH base acid Conj A Conj B. Chemistry activity of integration look at the poster the poster in fig. 6 What is the conjugate base of OH-.
Answer 1 of 3. And mass and charge are as always CONSERVED. It has one less H atom and one more charge.
So when we add a H to OH- it becomes H2O and both the opposite charges on H and OH- neutralise each other. In respect to this what is the conjugate acid of HS -. What is a conjugate acid base pair for the following equilibrium.
Has employed you as the marketing office. OH H H₂O. The stronger the acid or base the weaker the conjugate.
Because it has three unpaired electrons and is able to donate the pair of electrons to Lewis acids for covalent bond formation. The solution contains an equal number of h o ions and oh ions. O H2SO4 O SO4 O HSO4 O S042- O H2SO42-close.
Calculate the ph of a 025m solution of h3o 2. O2 is the conjugate base of OH. 3 Which of the.
The H₂O becomes OH. Weve got the study and writing resources you need for your assignments. Check Answer and Solution for above question from Chemistry in Equilibrium - Tardigrade.
HPO 4 2 is an acid and H 2. H 2 O l HPO 4 2 aq H 2 PO 4 aq OH aq a. Conjugate base of OH- is A H2O B H3O C H D O2-.
In the equation OH- is the conjugate base to the acid H2O because H2O donates a hydrogen ion to form OH- the conjugate base. And for the conjugate base we remove a proton and for the conjugate acid we ADD a proton. First week only 499.
Asked 1 day ago in Chemistry by Sowaiba 712k points What is the conjugate base of OH O H -. D The weaker the conjugate acid. The ice cream com filofilo ltd.
Hydrosulfuric acid is the conjugate acid of the hydrogen sulfite ion it dissociates into in solution. Conjugate base is formed by loss of H. B The stronger the conjugate base.
Solution for What is the conjugate base of HSO4-. It can act as an acid or a base. Look at your answer for 4 and 5 which one is a.
The two setsNH 3 NH 4 and H 2 OOH are called conjugate acid-base pairsWe say that NH 4 is the conjugate acid of NH 3 OH is the conjugate base of H 2 O and so forth. Additionally is hco3 an acid or base. H 2 O is an acid and OH is its conjugate base.
An acid and its conjugate base differ by one proton only. The hydroxide acts like a Bronsted Lory base so it can catch a proton. H 2O H 2 O.
Hence the conjugate acid for OH- is H20. So if we have to find a conjugate acid for a given base then we just have to add a H hydrogen ion to the given base. So OH is the conjugate base of H₂O.
H H O H 2Ol And thus the conjugate acid of hydroxide ion is simply water. When acid gives H then the remaining of its part is called conjugate base. A HPO 4 2-B PO 4 3-C H 3 PO 4.
According to Bronsted and Lowry theoryself generated acid base pair due to donation or acceptance of proton is known as conjugated acid base pairConjugated acids and bases differ by protonThus every acid has one excess proton than its conjugated baseIf OH- loses protonthen O2- ion is obtainedHence conjugated base of OH- is O2-. C The weaker the conjugate base. Calculate the ph of a 63x10-8m solution of h3o 3.
OH O 2H. None of the above e. HPO 4 2 is an acid and OH is its conjugate base.
The conjugate base of an acid is formed when the acid donates a proton. Water will be the conjugate acid. H₂O is an amphoterus compound.
1 What is the conjugate base of H 2 PO 4. So if we remove H from OH we get O 2. D H 3 O E OH 2 The stronger the acid then which of the following is true.
A The stronger the conjugate acid. Start your trial now. H 2 O is an acid and HPO 4 2 is its conjugate base.
So H₂CO₃ is the conjugate acid of HCO₃. The solution contains a larger number of h o ions than qh ions. Every Brønsted-Lowry acid-base reaction.
The solution contains a larger number of oh ions than h o ions. OH ion also acts as a Lewis base. E None of the above.

Equilibrium Constant Autoionization Of Water Science Chemistry Ap Chemistry Chemistry
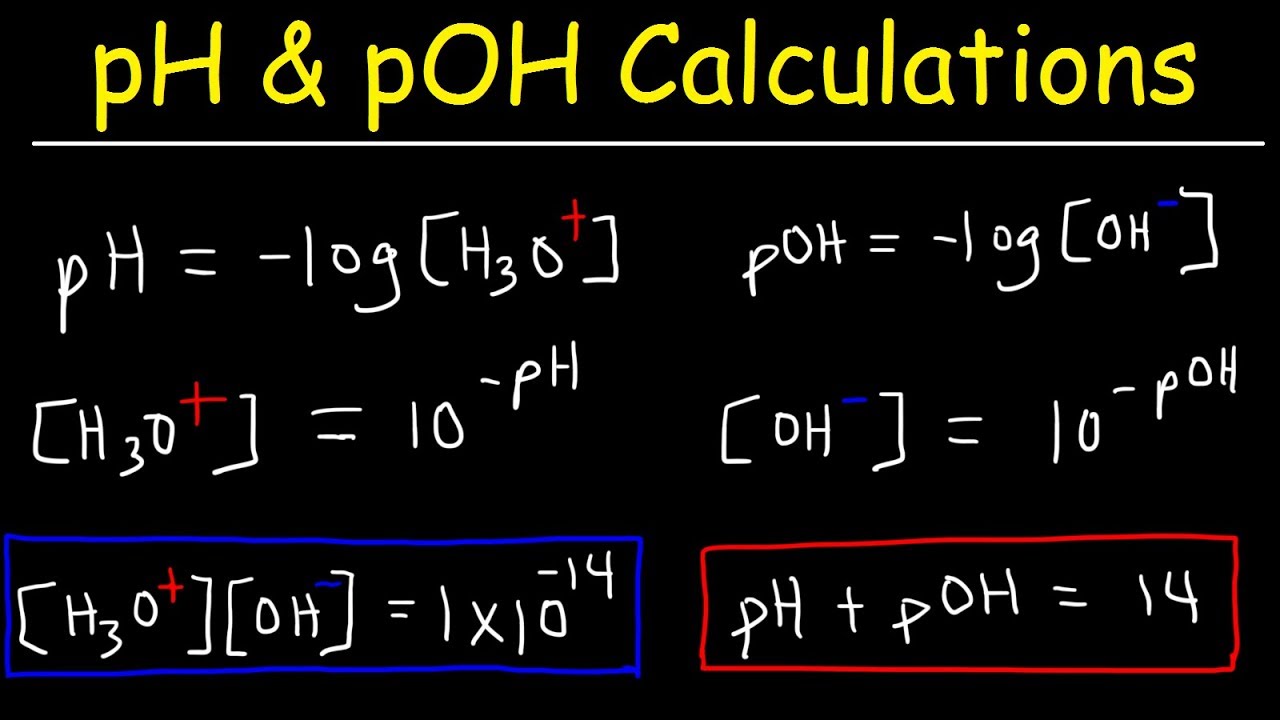
Ph Poh H3o Oh Kw Ka Kb Pka And Pkb Basic Calculations Acids And Bases Chemistry Problems Chemistry Lessons Chemistry Classroom Teaching Chemistry

Organic Chemistry Notes Carboxylic Acids Chemistry Notes Organic Chemistry Notes Organic Chemistry

Important Question For Class 10 Science Carbon And Its Compounds Https Www Learncbse In Carbon And Its Compounds Ch Chemistry Notes Chemistry Class Chemistry

Some Examples For Strong And Weak Acids And Bases

Grignard Reagent Is A Base Chemistry Lessons Chemistry Alcohol

E1cb Elimination Reaction Chemsolve Net Chemistry Reactions Organic Reactions

E1cb Elimination Reaction Chemsolve Net Reactions Map Chart

Pin On Acids And Bases In Organic Chemistry









Comments
Post a Comment